Understanding OECD 432:
In Vitro 3T3 NRU Phototoxicity Test
Phototoxicity (photoirritation) refers to toxic responses triggered by chemicals when the skin or eyes are exposed to light—especially ultraviolet (UV) radiation. It’s a critical endpoint for safety assessments in cosmetics, pharmaceuticals, and industrial chemicals. To reduce animal testing, the OECD Test Guideline 432 provides a scientifically validated, in vitro alternative: the 3T3 Neutral Red Uptake (NRU) Phototoxicity Test. The 3T3 NRU Phototoxicity Test boasts a sensitivity of 97.3%, a specificity of 86.7% and an accuracy of 94.2% (n=52) in the prediction of phototoxicity compared to human test results.
What Is the In Vitro 3T3 NRU Phototoxicity Test?
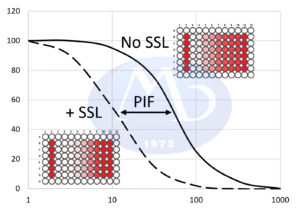
OECD TG 432 describes a method using cultured mouse fibroblast cells (BALB/c 3T3 cells) to evaluate a substance’s potential to cause photo-induced cytotoxicity. It compares the viability of test article treated cells (No SSL) to that of cells exposed to both the test article and simulated solar light (+ SSL).
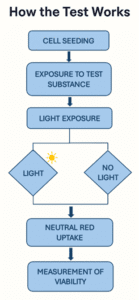
How the Test Works
- Cell Seeding Mouse 3T3 cells are seeded into microplates and cultured to adhere.
- Chemical Treatment A range of test concentrations is applied to the cells in duplicate plates. Control compounds (e.g. chlorpromazine) are evaluated in parallel.
- UVA Exposure
- One plate is exposed to calibrated UVA light.
- The other plate is kept in the dark as a non-irradiated control.
- Neutral Red Uptake (NRU) Assay Cells are incubated with Neutral Red dye, which viable cells absorb into lysosomes. The amount of dye uptake is measured spectrophotometrically, reflecting cell viability.
- Phototoxicity Evaluation Data from irradiated and non-irradiated cells are compared using:
- Photo Irritation Factor (PIF)
- Mean Photo Effect (MPE) Values exceeding defined thresholds indicate phototoxic potential.
Interpretation of Results
Criterion Interpretation
PIF ≥ 5 or MPE ≥ 0.15 Phototoxic
PIF 2–5 or MPE 0.1–0.15 Borderline
PIF < 2 and MPE < 0.1 Non-phototoxic
These thresholds help determine if a compound requires further testing or labeling for phototoxic risks.
Benefits of OECD 432
- Animal-free approach aligned with the 3Rs (Replacement, Reduction, Refinement)
- High sensitivity for UVA-active compounds
- Suitable for regulatory submissions in cosmetics and pharmaceuticals
- Standardized and reproducible
Limitations of OECD 432
- Not suitable for UVB-activated compounds or those requiring metabolism
- Limited applicability for highly colored or insoluble substances
Conclusion
The OECD TG 432 3T3 NRU Phototoxicity Test is a robust, in vitro method that helps identify phototoxic hazards without animal use. As photoreactivity remains a key concern for topical and systemic exposures, this test supports safer product development and regulatory compliance in a more ethical and efficient way.
Interested in adding a non-animal testing method to evaluate phototoxicity potential to your safety assessment plan? Contact MB Research Labs or explore our full suite of in vitro cytotoxicity tests.
Reference:
- Lee, Ga-Young; Hwang, Jee-Hyun; Hong, Jeong-Hyun; Bae, Seungjin; Lim, Kyung-Min (2025-06-28). “PhotoChem Reference Chemical Database for the Development of New Alternative Photosafety Test Methods”. Toxics. 13 (7): 545. doi:10.3390/toxics13070545. ISSN 2305-6304. PMC 12298655. PMID 40710990.