GLP OECD 442E h-CLAT
Human Cell Line Activation Test
Expert Non-Animal Skin Sensitization Testing
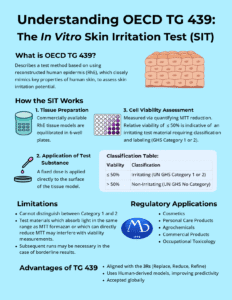
The Human Cell Line Activation Test (h-CLAT), described in OECD Test Guideline 442E, is a non-animal, in vitro test method used to identify the skin sensitization potential of chemicals. Conducted under Good Laboratory Practice (GLP) at MB Research Labs, the h-CLAT measures key immune cell activation events that occur during the skin sensitization process, helping regulatory agencies and product developers assess safety without the use of animals.
What Does the h-CLAT Measure?
The h-CLAT quantifies the expression of cell-surface activation markers CD86 and CD54 on THP-1 human monocyte cells following chemical exposure. These markers are upregulated during dendritic cell activation, a critical event in the Adverse Outcome Pathway (AOP) for skin sensitization.
By measuring these markers using flow cytometry, the test determines whether a chemical induces immune cell activation consistent with sensitizer classification under the Globally Harmonized System (GHS).
What is the Skin Sensitization Adverse Outcome Pathway (AOP)?
The skin sensitization AOP consists of four key biological events:
- Molecular Initiating Event (MIE) – covalent binding to skin proteins (measured by DPRA, OECD 442C)
- Key Event 2 (KE2) – keratinocyte activation (measured by KeratinoSens™, OECD 442D)
- Key Event 3 (KE3) – dendritic cell activation (h-CLAT, OECD 442E)
- Key Event 4 (KE4) – T-cell proliferation and response (evaluated by LLNA)
As part of a Defined Approach (DA) or Integrated Testing Strategy (ITS), h-CLAT complements other in vitro assays to provide mechanistically anchored, regulatory-accepted data for hazard classification.

What is the New Guidance: OECD Guideline No. 497 — Defined Approaches on Skin Sensitization?
The OECD Guideline No. 497 (2021) introduced internationally harmonized Defined Approaches (DAs) that combine data from multiple in vitro and in chemico assays to predict skin sensitization hazard and potency without animal testing.
These Defined Approaches integrate results from the DPRA (OECD 442C), KeratinoSens™ (OECD 442D), and h-CLAT (OECD 442E) using structured decision rules. When applied together, the DAs can classify substances as sensitizers or non-sensitizers and, in some cases, estimate potency (e.g., GHS 1A vs 1B).
Under OECD 497, h-CLAT plays a pivotal role as a cellular-response assay measuring immune cell activation. In combination with chemical reactivity (DPRA) and keratinocyte activation (KeratinoSens™), the h-CLAT enhances predictive accuracy, supporting weight-of-evidence or Defined Approach submissions accepted by global regulatory agencies including ECHA, EPA, and FDA.
MB Research Labs routinely integrates h-CLAT data into these OECD 497 frameworks to deliver comprehensive, regulatory-ready non-animal assessments.
GLP-Compliant h-CLAT Testing at MB Research Labs
MB Research Labs performs GLP-compliant OECD 442E h-CLAT studies to support regulatory submissions for:
- Industrial chemicals (REACH, EPA, TSCA)
- Cosmetic ingredients (EU Cosmetics Regulation)
- Medical device materials (ISO 10993-10 / 10993-23)
- Agrochemicals and specialty formulations
Our expert scientists follow strict Good Laboratory Practice (GLP) principles to ensure reliable, reproducible results and deliver comprehensive reports ready for regulatory review.
Why Choose h-CLAT for Skin Sensitization Screening?
✅ Animal-free – fully in vitro, human-cell based
✅ Mechanistically relevant – measures key immune cell activation event
✅ OECD & GLP compliant – globally recognized
✅ Quantitative & reproducible – flow-cytometry endpoints
✅ Compatible with integrated testing strategies
The h-CLAT offers an efficient, ethical, and scientifically robust alternative to traditional animal-based methods like the Local Lymph Node Assay (LLNA), Buehler Test, and the Guinea Pig Maximization Test (GPMT).
NAMs and Integrated Sensitization Testing at MB Research Labs
Our laboratory provides the full suite of OECD 442 series assays for skin sensitization:
- OECD 442C: Direct Peptide Reactivity Assay (DPRA)
- OECD 442D: KeratinoSens™ Assay (KS)
- OECD 442E: Human Cell Line Activation Test (h-CLAT)
Combined, these New Approach Methodologies (NAMs) form a complete non-animal strategy for sensitization hazard identification—accepted by OECD, EPA, ECHA, and FDA.
Partner with MB Research Labs
With three decades of GLP experience in both in vitro and in vivo sensitization testing, MB Research Labs is a trusted partner for regulatory-ready toxicology studies. Our experts can help you design an optimized testing strategy, interpret data across multiple assays, and meet the latest OECD and ISO 10993 requirements.
📩 Contact MB Research Labs to learn how our GLP h-CLAT and Defined Approach testing can streamline your non-animal skin sensitization assessments.